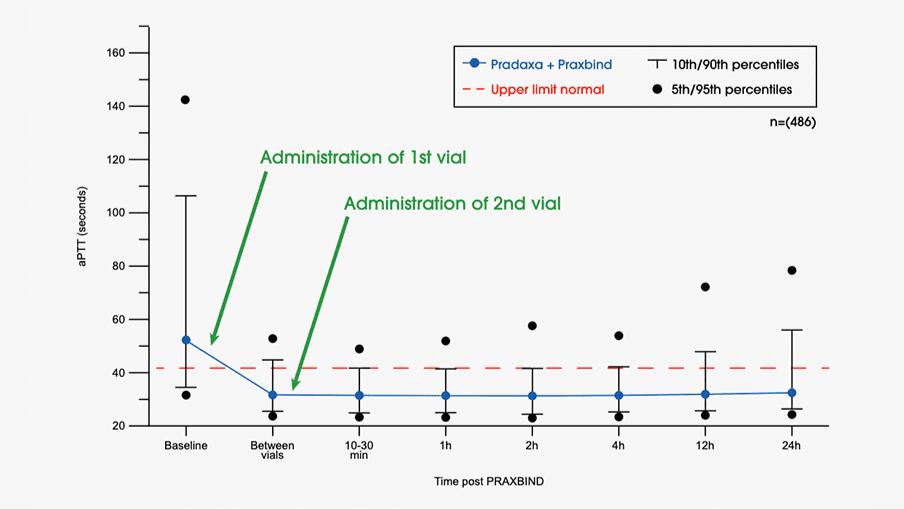
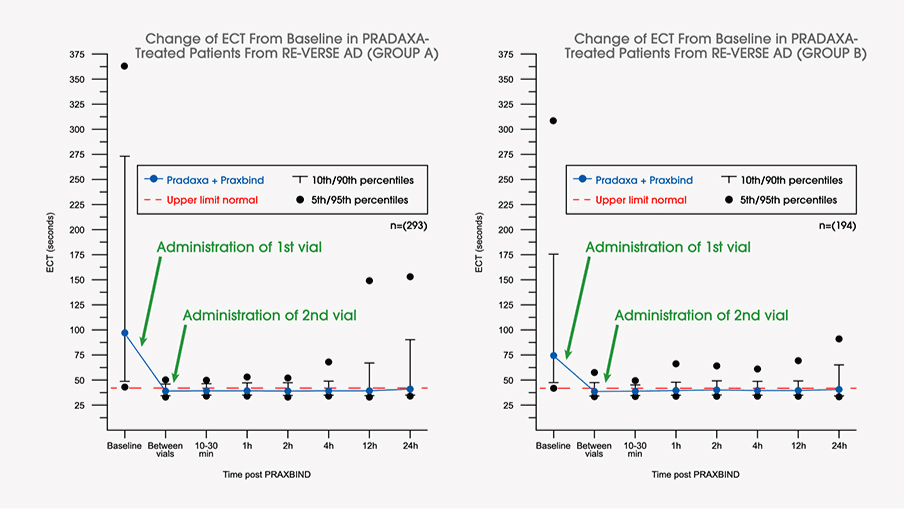
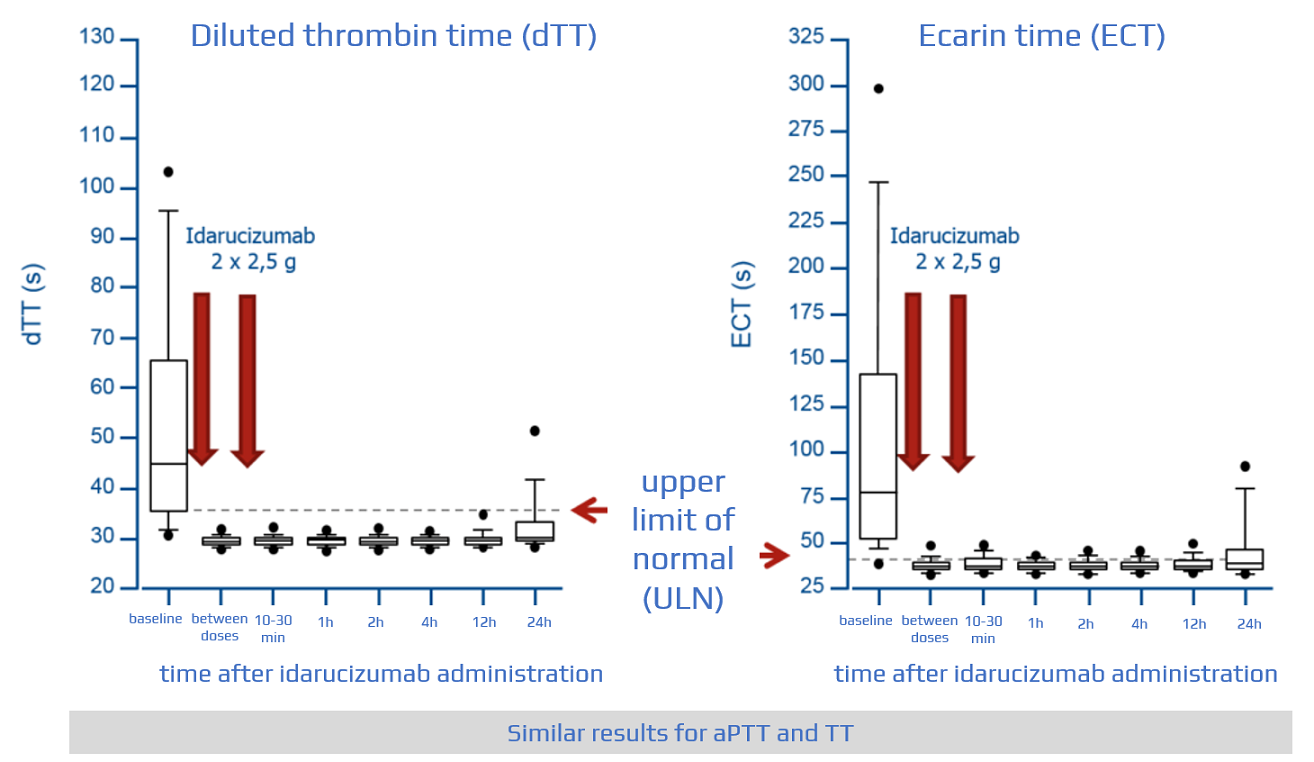
Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial - The Lancet

Idarucizumab and factor Xa reversal agents: role in hospital guidelines and protocols - ScienceDirect
Idarucizumab, a Specific Reversal Agent for Dabigatran: Mode of Action, Pharmacokinetics and Pharmacodynamics, and Safety and Ef

Idarucizumab, a Specific Reversal Agent for Dabigatran: Mode of Action, Pharmacokinetics and Pharmacodynamics, and Safety and Efficacy in Phase 1 Subjects - ScienceDirect
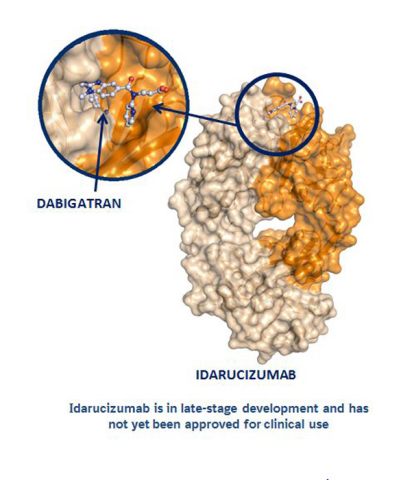
Boehringer Ingelheim submits applications for approval of Idarucizumab, an anti-anticoagulant - Labiotech.eu

FDA Approves Praxbind® (idarucizumab), Specific Reversal Agent for Pradaxa® (dabigatran etexilate) | Business Wire
Interpretation of idarucizumab clinical trial data based on spontaneous reports of dabigatran adverse effects in the French phar

Idarucizumab dosing in patients with excessive dabigatran body burden - British Journal of Anaesthesia

Dabigatran Reversal With Idarucizumab in Patients With Renal Impairment | Journal of the American College of Cardiology

PDF) Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials

Design of the Reversal Effects of Idarucizumab on Active Dabigatran... | Download Scientific Diagram
Usefulness of initial plasma dabigatran concentration to predict rebound after reversal | Haematologica

Medicina | Free Full-Text | Effectiveness and Safety of Dabigatran Reversal with Idarucizumab in the Taiwanese Population: A Comparison Based on Eligibility for Inclusion in Clinical Trials
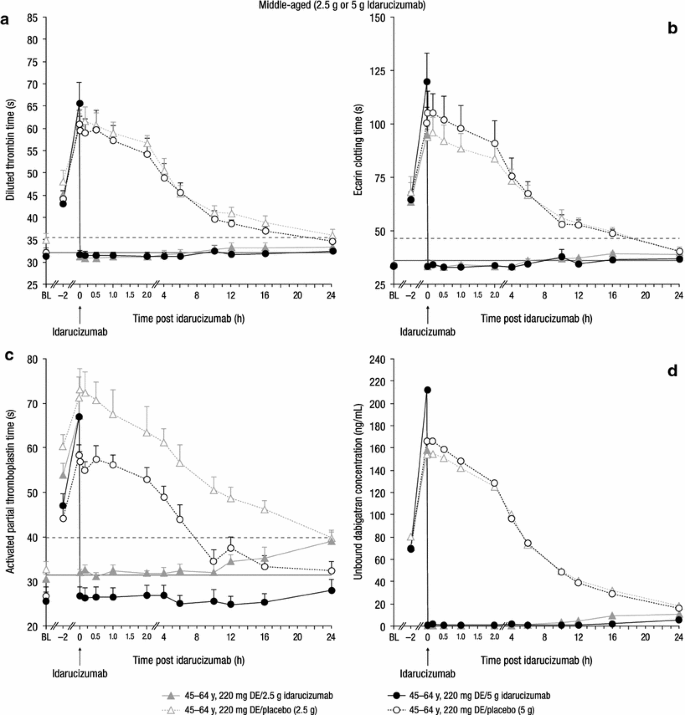
Effect of Age and Renal Function on Idarucizumab Pharmacokinetics and Idarucizumab-Mediated Reversal of Dabigatran Anticoagulant Activity in a Randomized, Double-Blind, Crossover Phase Ib Study | SpringerLink

Safety, tolerability, and efficacy of idarucizumab for the reversal of the anticoagulant effect of dabigatran in healthy male volunteers: a randomised, placebo-controlled, double-blind phase 1 trial - The Lancet