Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study - The Lancet

PDF) Abbreviated Clinical Study Reports with Investigational Medicinal Products for Human Use: Current Guidelines and Recommendations

Clinical Trial Report Template (6) - TEMPLATES EXAMPLE | TEMPLATES EXAMPLE | Report template, Clinical trials, Template google

Will clinical trial data disclosure reduce incentives to develop new uses of drugs? | Nature Biotechnology

Appendix F Illustrative Data Fields for the Results Summary | Developing a National Registry of Pharmacologic and Biologic Clinical Trials: Workshop Report | The National Academies Press
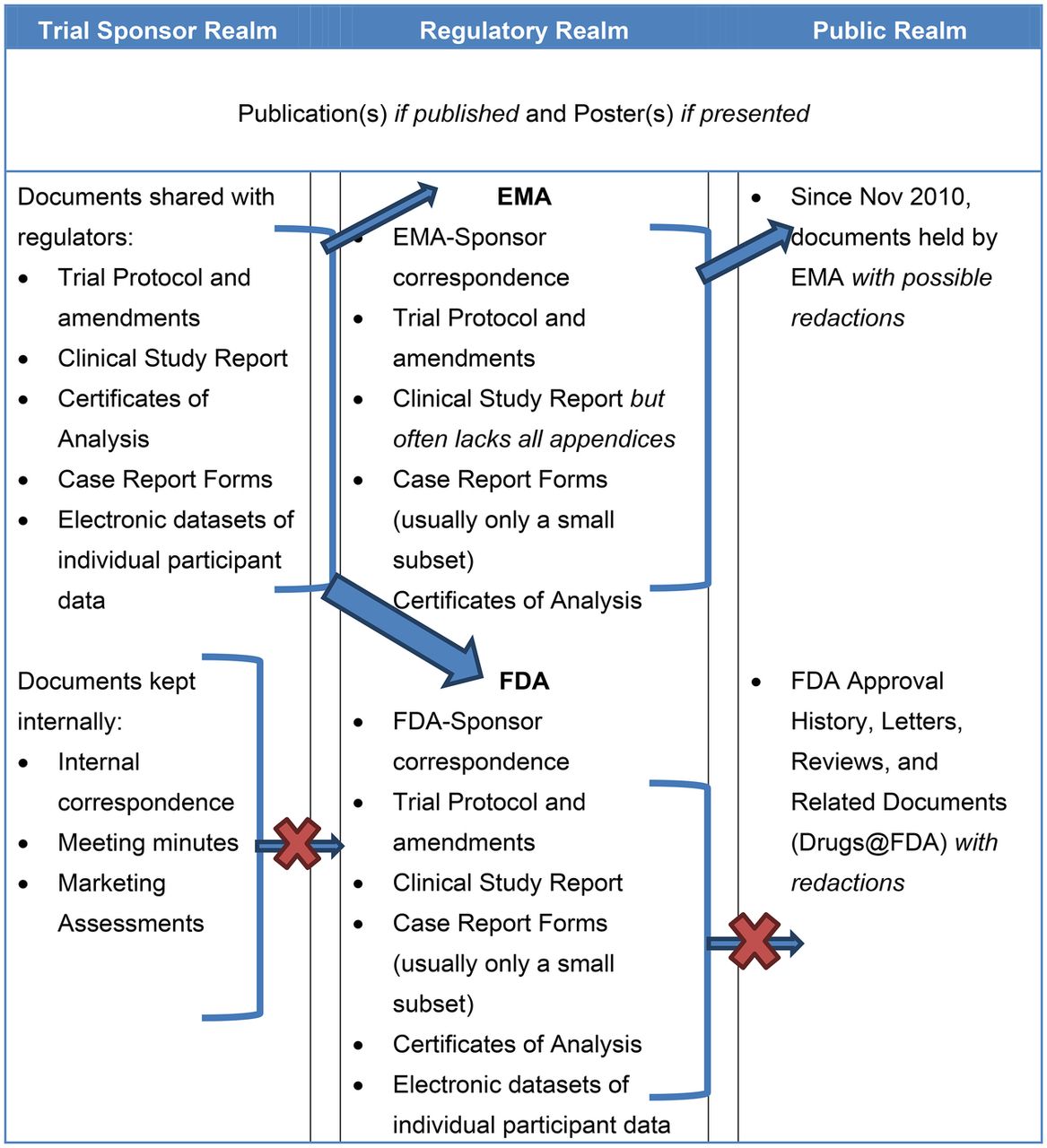
Completeness of Reporting of Patient-Relevant Clinical Trial Outcomes: Comparison of Unpublished Clinical Study Reports with Publicly Available Data | PLOS Medicine

Construction of study sample comprising the 50 most recent clinical... | Download Scientific Diagram

Appendix F Illustrative Data Fields for the Results Summary | Developing a National Registry of Pharmacologic and Biologic Clinical Trials: Workshop Report | The National Academies Press